Risks related to lowering DO in aerobic wastewater systems with respect to Ammonia and Total nitrogen removal
Lowering dissolved oxygen (DO) below 2 mg/L in aerobic wastewater treatment can reduce aeration energy costs, but it risks impairing nitrification (ammonia oxidation) and subsequent denitrification (nitrate/nitrite removal), leading to poor ammonia and total nitrogen (TN) removal. Certain influent characteristics exacerbate these risks by limiting microbial activity or oxygen penetration.
Biological Feasibility: Keeping NOB at 0.5 mg/L DO
The conventional wisdom is that AOB (Ammonia Oxidizers) outcompete NOB (Nitrite Oxidizers) at low DO because AOB have a higher affinity for oxygen. If you drop to 0.5 mg/L without other changes, you risk Nitrite Accumulation (partial nitrification).
To maintain NOB at this low level, you must shift the microbial community structure:
Select for Nitrospira over Nitrobacter
- High DO (2.0 mg/L): Favors Nitrobacter, which grows fast but has a poor affinity for oxygen ($K_O$ ≈ 0.5–1.0 mg/L).
- Low DO (0.5 mg/L): Favors Nitrospira. These are “K-strategists”—they grow slowly but have a very high affinity for oxygen.
- Strategy: You must gradually acclimatize the sludge to low DO to allow Nitrospira to become the dominant NOB.
Increase Sludge Retention Time (SRT)
Since low-DO adapted NOB (Nitrospira) grow very slowly, you must increase your SRT to prevent washing them out.
- Standard SRT: 10–12 days.
- Low DO SRT: Likely requires 15–20+ days (depending on temperature) to ensure the slow-growing NOB are retained.
The “Diffusion” Factor
At 0.5 mg/L bulk DO, the center of your activated sludge flocs will be anoxic. To maintain full nitrification, you may need to increase mixing energy slightly or manage F/M (Food to Microorganism) ratios to keep floc sizes smaller, ensuring oxygen can penetrate the biomass.
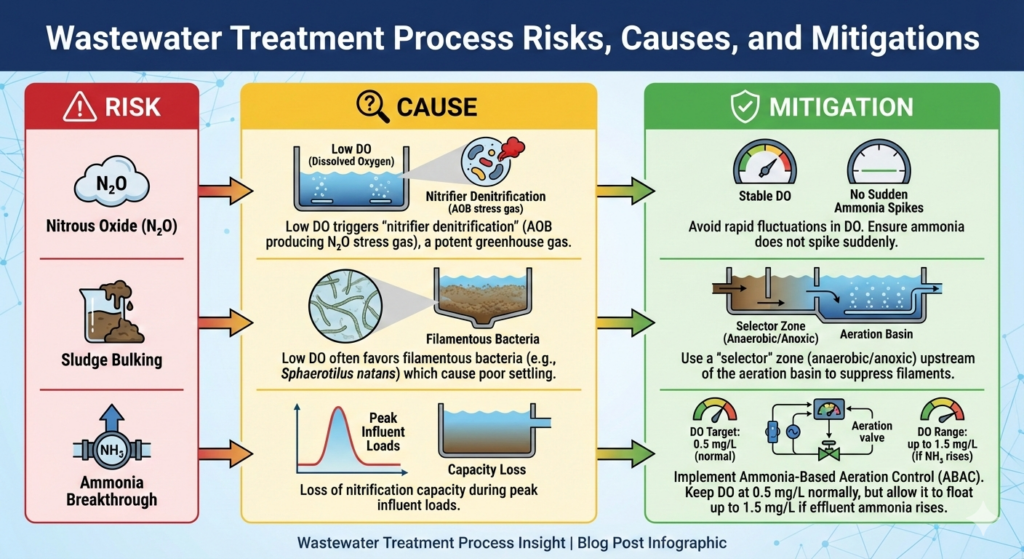
Operational Risks & Mitigation
| Risk | Cause | Mitigation |
| Nitrous Oxide (N₂O) | Low DO triggers “nitrifier denitrification” (AOB producing N₂O stress gas). This is a potent greenhouse gas. | Avoid rapid fluctuations in DO. Ensure ammonia does not spike suddenly. |
| Sludge Bulking | Low DO often favors filamentous bacteria (e.g., Sphaerotilus natans) which cause poor settling. | Use a “selector” zone (anaerobic/anoxic) upstream of the aeration basin to suppress filaments. |
| Ammonia Breakthrough | Loss of nitrification capacity during peak influent loads. | Implement Ammonia-Based Aeration Control (ABAC). Keep DO at 0.5 mg/L normally but allow it to float up to 1.5 mg/L if effluent ammonia rises. |
Where to Use Caution with Lowering D.O. Residuals
High Influent Ammonia-Nitrogen Concentrations
High ammonia loads (e.g., >40-50 mg/L NH₄⁺-N) increase oxygen demand, as nitrification theoretically requires ~4.57 mg O₂ per mg NH₄⁺-N oxidized. At low DO (<2 mg/L), oxygen diffusion into sludge flocs becomes limiting, slowing ammonia-oxidizing bacteria (AOB) activity and causing ammonia breakthrough in effluent. High-strength wastewaters (e.g., industrial or sidestream) often face incomplete nitrification without sufficient DO.
Low Carbon-to-Nitrogen (C/N) Ratio
Low influent COD/N or BOD/N ratios (e.g., <4-5:1) limit organic carbon for heterotrophic denitrifiers. Even if nitrification occurs, excess nitrate accumulates due to incomplete denitrification. At low DO, heterotrophs compete aggressively with nitrifiers for oxygen, further suppressing AOB and NOB (nitrite-oxidizing bacteria). Low C/N wastewater (common in municipal systems diluted by rainwater) often requires external carbon addition for reliable TN removal under low-DO conditions.
Insufficient Influent Alkalinity
Nitrification consumes ~7.14 mg/L alkalinity (as CaCO₃) per mg/L NH₄⁺-N oxidized, producing acidity that drops pH. Low influent alkalinity (<100-150 mg/L) fails to buffer this, causing pH to fall below 6.8-7.0, where nitrifiers become inhibited. Low DO worsens this by slowing nitrification rates, prolonging acid production and risking partial or failed ammonia removal.
Low Wastewater Temperature
Cold influent (<15-20°C, especially in winter) slows nitrifier growth rates dramatically (doubling time increases exponentially below 20°C). Low DO compounds this by reducing oxygen availability when microbial kinetics are already sluggish, often leading to seasonal nitrification failure and high effluent ammonia/TN.
Presence of Inhibitors or Toxic Compounds
Influent containing heavy metals, organic compounds (e.g., from industrial sources), or high salinity inhibits slow-growing nitrifiers more than heterotrophs. Low DO amplifies toxicity effects by stressing oxygen-limited AOB/NOB, resulting in poor nitrogen removal.
High Organic Loading with Low Biodegradable Fraction
High total COD but low readily biodegradable fraction (rbCOD) limits carbon availability for simultaneous nitrification-denitrification in low-DO systems. Heterotrophs outcompete nitrifiers for limited oxygen, suppressing nitrification.
Practical Implications and Mitigation
These characteristics often interact (e.g., cold + low alkalinity + high ammonia). Plants with favorable influent (moderate ammonia ~20-40 mg/L, C/N >5-6, alkalinity >150 mg/L, temperature >20°C) can sustain low-DO operation (0.5-1.5 mg/L) with advanced controls like ammonia-based aeration or biofilm systems. For challenging influents, maintain DO >2+ mg/L, add alkalinity/carbon, or use hybrid processes (e.g., partial nitritation-anammox) tolerant of low DO.
