Quaternary Ammonium Compounds (QACs) are commonly found in disinfectants, detergents, and personal care products. Their extensive use has led to increased concentrations in wastewater. To better understand how this impacts biological wastewater treatment, let’s examine how QACs interact with the biomass and their environmental fate.
What Exactly Are a QACs
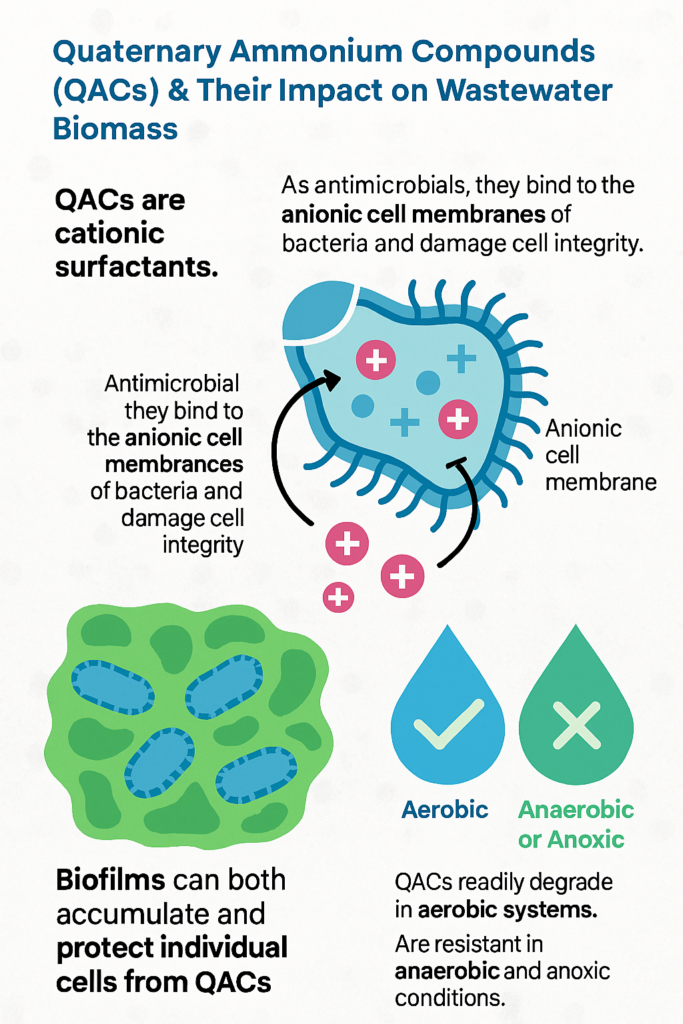
Quaternary Ammonium Compounds (QACs) are cationic surfactants commonly found in disinfectants, consumer products, and detergents. The chemical structure of QACs, which includes a positively charged nitrogen atom with attached hydrophobic hydrocarbon chains, gives them a strong affinity for negatively charged surfaces like microbial cells.
QAC as Disinfectants & Antimicrobials
Quaternary ammonium compounds (QACs) disrupt and kill bacterial cells by targeting their cell membranes. These compounds are positively charged surfactants that interact with the negatively charged bacterial membrane, leading to membrane destabilization. This causes leakage of vital intracellular contents, loss of membrane potential, and ultimately cell lysis. QACs also interfere with protein function and enzyme activity, further compromising bacterial viability. Their broad-spectrum antimicrobial action makes them effective disinfectants, especially against Gram-positive bacteria.
QAC has a proportionally higher impact on sensitive nitrifying bacteria which grow on the biofilm (floc) surface in areas with higest D.O.. High concentrations of QAC can inhibit the biological treatment process, slowing down the degradation of QACs and other pollutants. Additionally, QACs can accumulate in biofilms, contributing to toxicity toward microorganisms in the biofilm. However, biofilms often exhibit reduced susceptibility to QACs compared to free-floating cells.
Impact of QACs on Wastewater Biomass
This interaction can disrupt cell membranes, leading to cell lysis and death. Additionally, QACs can become toxic to microorganisms at high concentrations, particularly affecting sensitive nitrifying bacteria, which can inhibit the biological treatment process and slow down the degradation of QACs and other pollutants.
Affinity for negative charges (anonic) in the biomass is the first step in both QAC microbial inhibition and removal from the wastewater. QAC fate is a combination of sorption and degradation, with sorption being the most rapid and dominant initial removal mechanism.
Biochemistry of QAC Degradation
Like other surfactants, QACs undergo biodegradation primarily under aerobic conditions in wastewater treatment systems. Many QACs are considered ultimately biodegradable in aerobic biological treatment, particularly after they have adsorbed to the sludge solids. The rate and extent of biodegradation depend on the specific QAC structure.
Key bacterial genera involved in QAC degradation include Pseudomonas, Xanthomonas, and Aeromonas, which metabolize QACs through enzymatic pathways such as hydroxylation, oxidation, and dealkylation. Under anoxic or anaerobic conditions, QACs are recalcitrant and tend to accumulate rather than degrade.
Effective Management of Wastewater Treatment Systems
Understanding the biochemistry of QAC degradation and toxicity is crucial for managing wastewater treatment systems effectively. Factors such as chemical structure, oxygen availability, plant operational parameters, microbial community, and interactions with other agents influence QAC biodegradation. For instance, shorter alkyl chains favor biodegradation due to higher bioavailability, while longer chains promote sorption over degradation. Aerobic conditions are essential for effective degradation, and plant operational parameters like longer solids retention time (SRT) and hydraulic retention time (HRT) enhance degradation. While QACs significantly impact wastewater biomass through sorption and accumulation in sludge. By optimizing conditions for biodegradation and mitigating toxicity, we can improve the efficiency of QAC removal and ensure the sustainability of wastewater treatment processes.
